Chemistry is full of compounds that may seem complex at first glance but reveal fascinating details when studied closely. One such example is hcooch ch2 h2o, a combination of chemical notations that represents essential interactions in organic and inorganic chemistry. This keyword points to methyl formate (HCOOCH₃), the methylene group (CH₂), and water (H₂O), three important elements in the study of chemical reactions, synthesis, and applications. Exploring this combination allows us to better understand how molecules behave, how they react in different environments, and why they are so important in both laboratory and industrial use.
Breaking Down hcooch ch2 h2o
When we look at hcooch ch2 h2o, we are essentially analyzing three parts that come together in scientific studies
- HCOOCH₃ (methyl formate) An ester formed from methanol and formic acid.
- CH₂ (methylene group) A common structural unit in organic molecules.
- H₂O (water) The universal solvent and essential reactant in hydrolysis and other reactions.
Together, these elements illustrate reactions where organic esters interact with water in the presence of functional groups like CH₂. This makes hcooch ch2 h2o a valuable point of study for chemists working on synthesis, hydrolysis, or chemical engineering applications.
Properties of hcooch ch2 h2o Compounds
The individual components of hcooch ch2 h2o have unique properties that influence their behavior in reactions.
- Methyl formate (HCOOCH₃)
- Colorless liquid with a pleasant odor.
- Boiling point around 31–34 °C.
- Soluble in water and many organic solvents.
- Flammable and volatile.
- CH₂ group
- Exists within organic chains as –CH₂–.
- Highly reactive in radical or carbene forms.
- Plays a central role in building larger carbon-based structures.
- Water (H₂O)
- Universal solvent.
- Involved in hydrolysis, condensation, and acid-base chemistry.
- Stabilizes many reactions and provides the medium for chemical transformations.
The combination of these properties explains why hcooch ch2 h2o is central to many laboratory and industrial studies.
Reactions Involving hcooch ch2 h2o
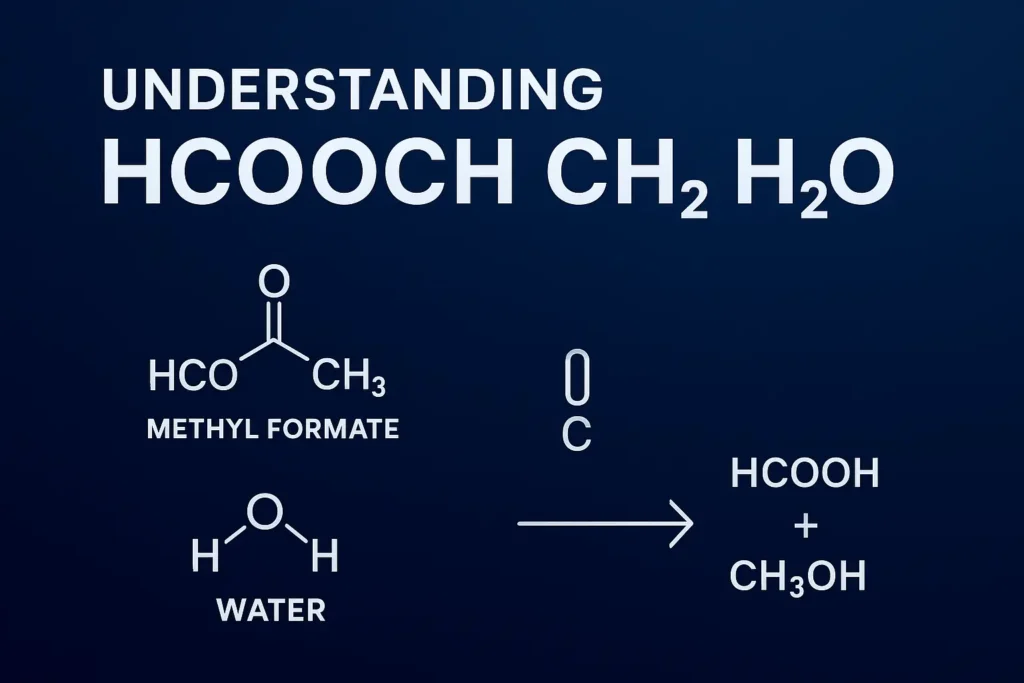
The most significant reaction involving hcooch ch2 h2o is hydrolysis. When methyl formate interacts with water, the following occurs
HCOOCH₃ + H₂O → HCOOH (formic acid) + CH₃OH (methanol)
This transformation demonstrates how an ester can break down into its parent alcohol and acid. The CH₂ group plays an indirect role, appearing in organic reaction mechanisms and pathways. Such reactions are widely studied in organic chemistry and have applications in the production of solvents, intermediates, and synthetic chemicals.
Industrial Applications of hcooch ch2 h2o
The study of hcooch ch2 h2o is not just theoretical; it has real-world applications across industries:
- Chemical Manufacturing: Hydrolysis of methyl formate is used in producing formic acid and methanol, both of which are valuable industrial chemicals.
- Solvent Use: Methyl formate acts as a solvent in coatings, adhesives, and extraction processes.
- Pharmaceuticals: CH₂ groups are part of drug structures, and reactions involving water and esters form the basis of many synthesis pathways.
- Polymer Industry: Water and ester reactions contribute to processes in plastics and foams, where methyl formate is sometimes used as a blowing agent.
- Environmental Relevance: Understanding hydrolysis helps in analyzing how esters break down in nature, which is important for pollution control and environmental safety.
The Role of hcooch ch2 h2o in Organic Chemistry
Organic chemistry often revolves around the behavior of small groups like CH₂, functional molecules like esters, and the ever-present role of water. The combination of these elements in hcooch ch2 h2o provides an excellent example of how molecular structures and reactions shape the chemical world. Whether it is learning about ester hydrolysis in classrooms or applying it in research labs, this set of molecules bridges the gap between theory and application.
Importance in Scientific Research
Researchers use hcooch ch2 h2o studies to:
- Explore reaction mechanisms in detail.
- Understand how functional groups interact with solvents.
- Model chemical processes that are relevant in larger-scale production.
- Train students in practical chemistry through simple but powerful reactions.
The value of hcooch ch2 h2o lies in its simplicity, yet its ability to explain key concepts in chemistry that are widely used in academia and industry.
Conclusion
The study of hcooch ch2 h2o highlights how fundamental compounds like methyl formate, methylene groups, and water come together to demonstrate key principles of chemistry. From hydrolysis reactions to industrial applications, this topic provides a rich field of knowledge that connects science to real-world uses. By understanding this combination, learners and professionals gain insights into chemical behavior, synthesis pathways, and industrial relevance.
FAQs
1. What does hcooch ch2 h2o represent?
It refers to methyl formate (HCOOCH₃), the methylene group (CH₂), and water (H₂O), studied together in chemical reactions.
2. What reaction occurs with hcooch ch2 h2o?
The main reaction is hydrolysis, where methyl formate reacts with water to form methanol and formic acid.
3. Why is hcooch ch2 h2o important in industry?
It is important because it explains ester hydrolysis, used in producing solvents, chemicals, and intermediates.
4. Is hcooch ch2 h2o used in environmental studies?
Yes, hydrolysis reactions are studied to understand how esters break down in natural environments.
5. How does CH₂ relate to hcooch ch2 h2o?
CH₂ is part of organic structures and plays a role in reactions involving esters and water, making it central to organic chemistry studies.